3 innovative ways to generate real world evidence
Real world evidence, i.e. knowledge gained from real world data, is a booster for the pharmaceutical industry in the development, authorisation and marketing of its products. Today, they have better data sources than ever for clinical studies. We present 3 innovative possibilities in this article.
The term Real World Data (RWD) is associated with a great hope for the pharmaceutical industry: large amounts of health data that give clinical research access to new knowledge about diseases and drugs - and develop an innovative power for the entire healthcare system. The magic word here is: Real World Evidence (RWE) - in other words, scientific findings obtained from real-world data.
Real World Data: What's behind it?
Real world data is health data that is collected from patients in their everyday lives. This can be data collected via electronic patient records (e-health records) or digital measuring devices and applications, for example. The advantage of RWD is that it reflects the health and behaviour of people in their natural environment. Compared to data collected as part of clinical studies under highly controlled conditions, RWD is therefore a much better reflection of a patient's reality in everyday care.

At the same time, RWD is high quality data that gives researchers a more accurate picture of how drugs work in different patient groups. With the help of the data obtained from RWD Findings on the effect, adherence and efficacy of medication in the real care situation (Real World Evidence), therapies can be tailored much better to individual patients. RWDs are therefore one of the beacons of hope on the road to personalised medicine that focuses on the individual patient.

The reality of clinical research is currently still a long way from realising the potential of RWD. Because in order to generate real-world evidence, you need Structured, high-quality data in digital form. However, paper and laboriously created Excel spreadsheets are still frequently used as data carriers in research projects. Yet there are simple digital methods for continuously, conveniently and efficiently recording valuable real-world data in patients' everyday lives.
Generate RWE: You should know these 3 possibilities
1.
Remote Patient Monitoring (RPM)

A continuous course of central vital signs (e.g. heart or lung function) is a decisive factor in evaluating the success of a therapy. It shows how a patient's state of health develops over time - e.g. as a result of taking a medication. However, regular visits as part of a clinical trial are a time-consuming process that limits the number of measurement points and therefore only provides selective snapshots of the overall picture. However, if the subjects record their values themselves and enter them in forms, the data density often suffers from suboptimal measurement adherence.
Remote patient monitoring (e.g. with our RPM platform SaniQ) is able to do this, RWD with very high data density and the highest data quality in real time to generate. With RPM, patients are medically monitored outside the clinical facility using digital technology. Thanks to daily measurement points, researchers receive a consistent picture that provides valuable insights into the effect of a drug. Measurement and medication adherence can also be positively influenced with RPM, for example by using a patient app to remind the test subjects of due measurements or intake.
2.
Sensor technology: wearables & co.

The use of wearables (e.g. smartwatches) or other electronic sensor devices (e.g. blood pressure monitors, spirometers, etc.) is an efficient way to generate RWD. The sensor devices record health parameters such as pulse, heart rate or activity level and transmit them to a smartphone app or web-based software.
The advantage is obvious: the sensor devices generate continuous RWD in real time, which enables researchers to complete data record over a longer period of time to collect data. This allows events and fluctuations that might go unnoticed during sporadic visits to be recorded and analysed. In conjunction with remote patient monitoring, wearables & co. therefore stand for modern, digital recording of high-quality real-time data.
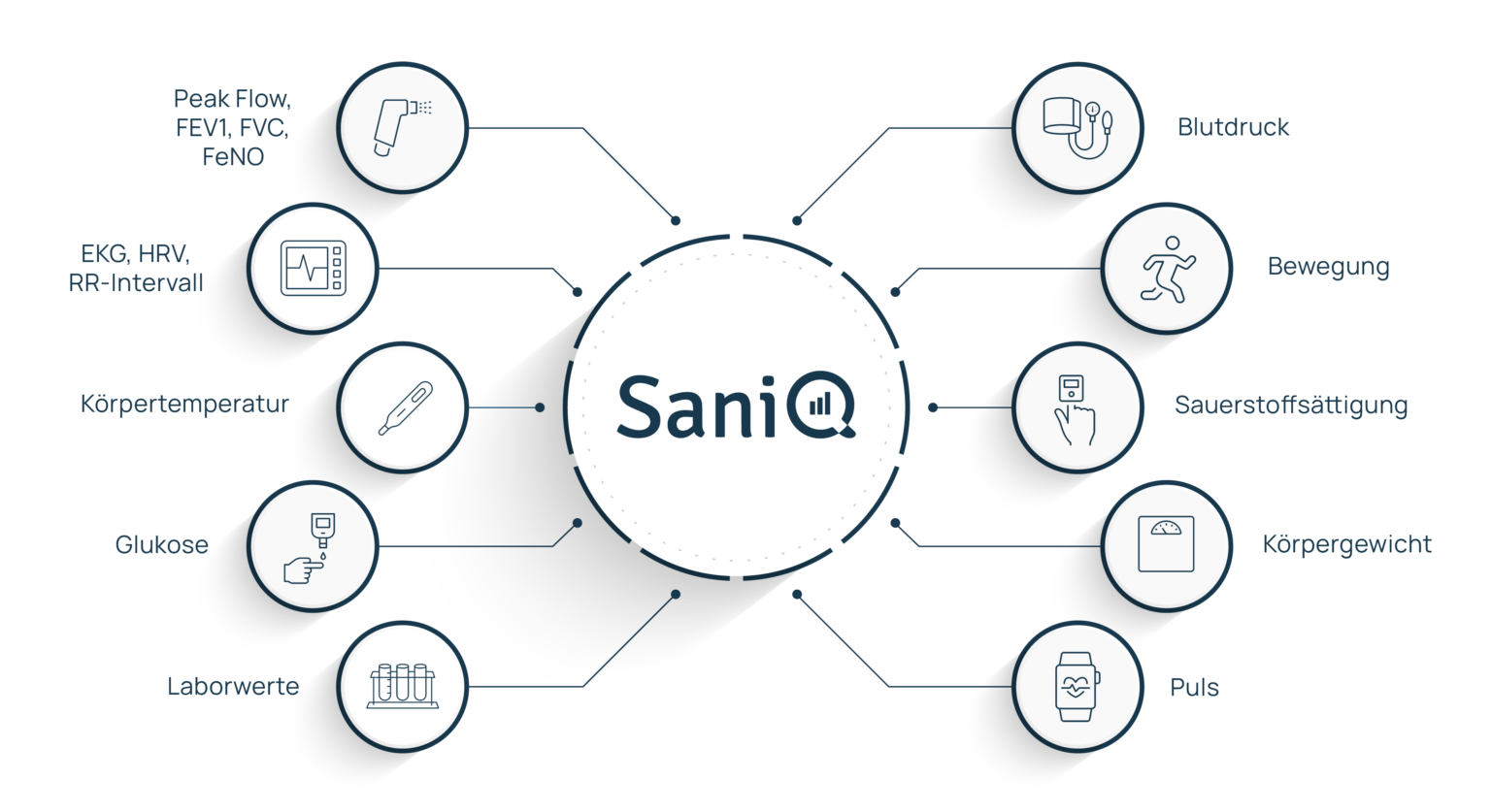
With our adaptable SaniQ platform, you can monitor more than 15 vital signs using a wide range of sensor devices:
3.
Electronic questionnaires (PREMs & PROMs)

The subjective experiences of patients with a therapy are among the most relevant real-world data. It provides pharmaceutical manufacturers with important information about the safety of a drug and adherence. When generating such data, however, pharmaceutical companies repeatedly encounter difficulties when it comes to reaching patients for regular surveys. In addition, questionnaires on paper are still frequently used to conduct such surveys, which then have to be laboriously transferred to a database.
Electronic questionnaires such as PREMs (Patient Reported Experience Measures) and PROMs (Patient Reported Outcomes Measures) offer a smart solution here by collecting patient feedback in a user-friendly digital way. Via the patient app of our RPM platform SaniQ For example, patients receive a notification as soon as a new questionnaire needs to be completed. This reminder function automatically prompts patients to fill out the questionnaire and eliminates the time-consuming process of transferring paper to the database.
Conclusion
Real world evidence has great potential for clinical trials and medical patient care. Pharmaceutical manufacturers can use it to develop better active ingredients, accelerate clinical trials and regulatory processes and better tailor therapy to individual patients in the sense of personalised medicine. Digital solutions such as our RPM platform SaniQ open up new opportunities for researchers in the pharmaceutical sector to generate RWE efficiently and in a patient-friendly way.
Let's talk about your project idea!

Jonas Zimmer
-Project development-
Let's talk about your project idea!
- Personal counselling
- Individual topic focus
- Discover advantages
