With real-world data to clinical evidence
With real-world data to clinical evidence
Clinical research needs real life data - SaniQ TRIAL transforms every day of study participation into valuable real world data and clinical evidence.


3 Challenges in clinical studies:
3 Challenges in clinical trials
Generate real-world data
Reduce the dropout rate
Communicate efficiently
How SaniQ TRIAL supports you:
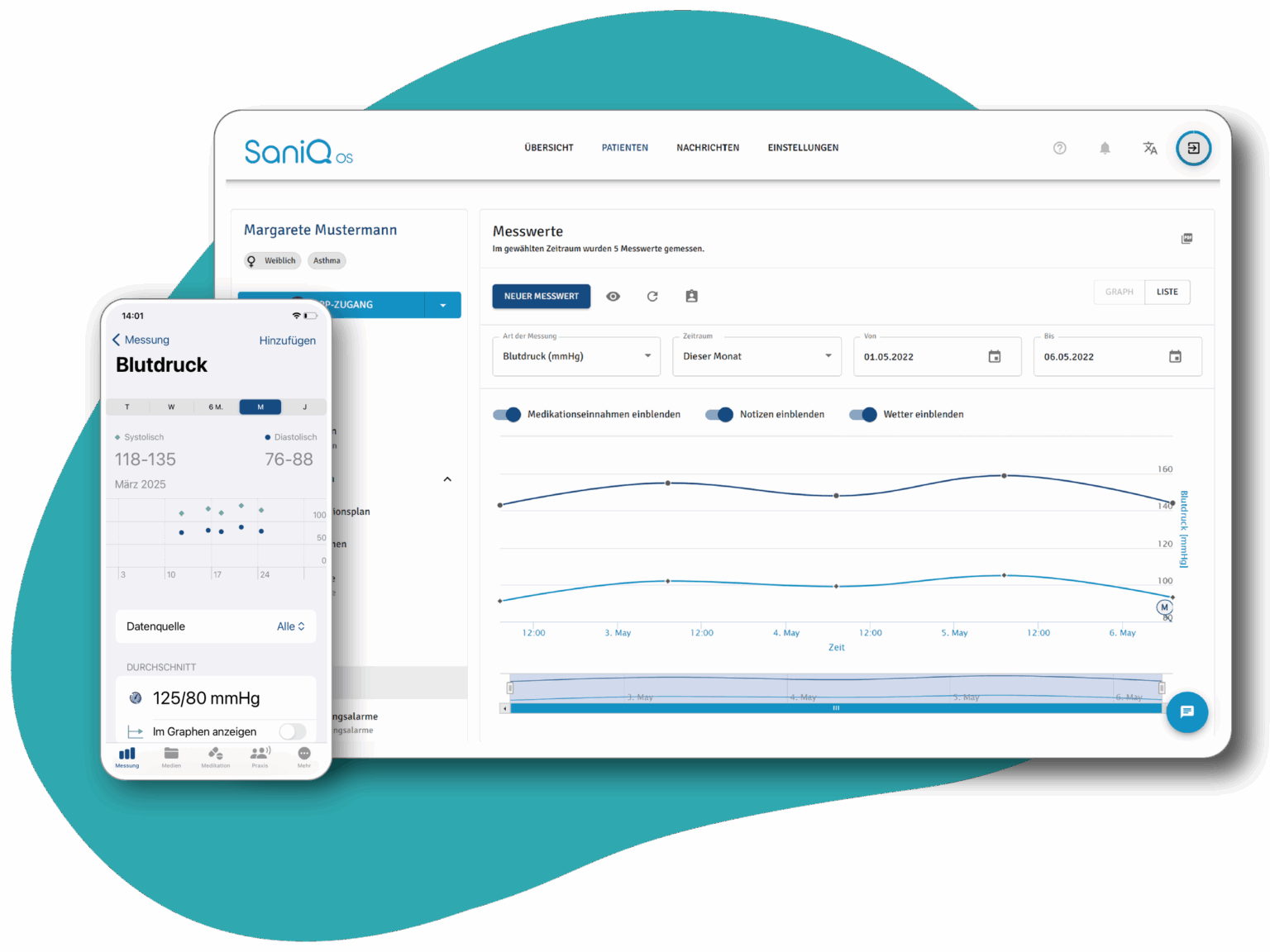
SaniQ TRIAL turns every day of study participation into valuable real-world data that you can track in real time via the SaniQ Dashboard.
1. continuous data
- Telemonitoring of vital signs
- Electronic questionnaires (PREMs & PROMs)
- Medication planner incl. intake times
2. better compliance
- Patient app for the active involvement of test subjects
- Medication planner incl. reminder function in the app
- Convenient and secure communication via televisite & chat
3. efficient therapy control
- Automated monitoring alarms
- Quick contact via chat & televisite
- Adjustment of the dosage in the medication planner
Smart solutions for efficient clinical trials
Smart solutions for efficient clinical trials
|
Modules & functions
|
Benefits for clinics
|
|---|---|
|
Telemonitoring (vital signs such as blood pressure, weight, FEV1, etc.)
|
Continuous data collection for study endpoints, reduction of study cancellations through better compliance
|
|
Workflow Manager
|
Structured distribution of tasks between trial centre, CRO and investigators
|
|
Intersectoral communication
|
GDPR-compliant exchange between different stakeholders
|
|
Patient app
|
Active involvement of study participants in the course of the study and improvement of adherence
|
|
Chat & Televisite
|
Direct contact in the event of side effects, queries or protocol-relevant events
|
|
EDC interfaces (CDISC, CDASH, etc.):
|
Seamless integration into existing study management systems and electronic data capture systems
|
1. continuous data
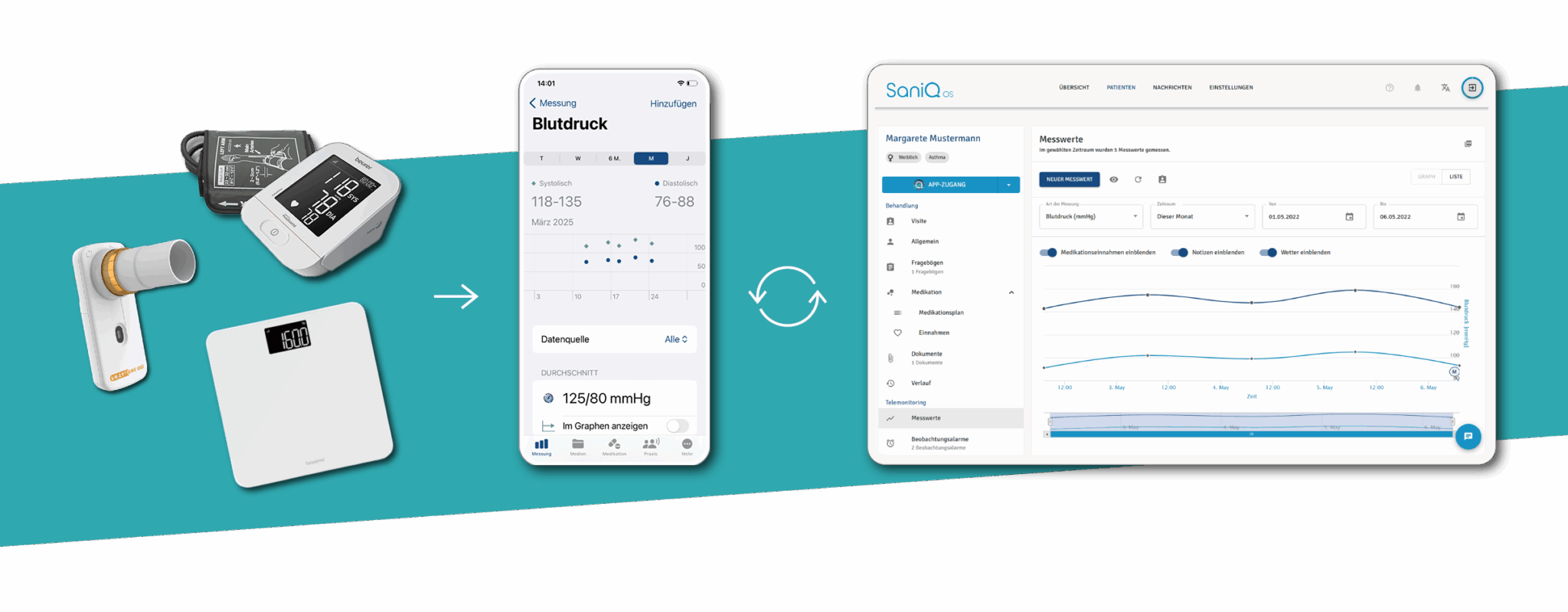
This is how you accompany test subjects through their everyday life with SaniQ:
- In the morning, they measure their vital signs at home using sensors connected to SaniQ.
- At lunchtime, they answer digital questionnaires about their symptoms via the SaniQ patient app.
- In the evening, they document how the new therapy affects their quality of life.
SaniQ transforms every day of study participation into valuable real-world data that you can track in real time via the SaniQ Dashboard.
What used to be recorded as isolated data points in sterile practice rooms becomes a vivid picture of the course of symptoms in everyday life.

2. better compliance
SaniQ makes study participation much more convenient for test subjects:
They measure their vital signs from the comfort of their own home, answer digital questionnaires (PREMs & PROMs) when it suits them and receive gentle reminders via the patient app if they forget something. Should any questions arise, they can communicate immediately via the platform via chat or video consultation instead of waiting for the next appointment.
This flexibility means that significantly fewer participants terminate the study prematurely and the data quality increases noticeably due to more consistent participation.

3. more efficient therapy control
Make treatment decisions when they are needed:
SaniQ notifies you directly when a test subject sends abnormal values. With just a few clicks, you can then view the current vital signs, evaluate questionnaire data and discuss the next steps via chat or video consultation without the patient first having to make an appointment or travel to the centre.
This ability to react quickly makes it possible to adapt the therapy efficiently and precisely to the course of symptoms and at the same time ensure complete documentation for the study data.

- CE-approved Class I medical device
- GDPR-compliant data protection and ISO 27001-certified
- Standardised interfaces to your HIS (HL7, FHIR)
-
CE-approved
Class I medical device -
GDPR-compliant data protection
and certified according to ISO 27001 -
Standardised interfaces
to your HIS (HL7, FHIR)
Use case: Telementor COPD study
The Telementor COPD study, funded by the G-BA Innovation Fund, is using SaniQ TRIAL to investigate the benefits of telemedicine for patients with COPD.
Period: 2022-2025
Partner: Lungenclinic Großhansdorf, Astra Zeneca, University Medical Centre Schleswig-Holstein, University Medical Centre Hamburg-Eppendorg, and many more.
Number of participants in the study: 640
Content: The Telementor COPD study is a multicentre, randomised and controlled clinical trial funded by the G-BA Innovation Fund under Consortium management of the LungenClinic Großhansdorf. The aim of the study is to prevent exacerbations in COPD patients through digital prevention and home monitoring.
Link to the study website: https://www.telementor-copd.de/
As part of the study, various vital signs of the patients are monitored using SaniQ OS and connected sensors (telespirometer, smartwatch) and analysed by the study staff:
- Telemonitoring lung function (FEV1), heart and respiratory rate, blood-oxygen saturation and daily step count for continuous patient monitoring
- API connection to the hospital information system for automated and bidirectional transmission of patient-related data
- Patient app to actively involve patients in the care programme and to generate patient-reported outcomes
- The study personnel regularly Televisits with the connected patients through
ZDF Berichtet über COPD Studie mit SaniQ
Das ZDF hat über die Telementor-COPD-Studie berichtet, bei der unsere Telemonitoring-Plattform SaniQ OS zur Überwachung von COPD-Patienten zum Einsatz kam.
to the article
Studies with SaniQ OS
SaniQ has already been used profitably in numerous clinical studies and research projects:

Telemonitoring for COPD in standard care?
Telementor-COPD is a multicentre, randomised and controlled clinical study funded by the Federal Joint Committee and led by LungenClinic Großhansdorf. With the help of SaniQ, it is investigating the benefits of telemonitoring for patients with COPD.

Telemedicine closes care gap
A team of doctors at Berlin's Charité hospital is breaking new ground to provide much-needed support to those affected by a neurological disease.
What researchers say about SaniQ TRIAL



Let's talk about your project idea!

Jonas Zimmer
-Project development-
Let's talk about your project idea!
- Personal counselling
- Individual topic focus
- Discover advantages