Mit kontinuierlichen Daten zu besseren Studien
Klinische Forschung braucht Daten aus dem echten Leben – nicht nur aus kontrollierten Studienumgebungen. Erfassen Sie mit der Telemedizin-Plattform SaniQ OS kontinuierlich hochwertige Real World Daten aus dem Alltag Ihrer Proband:innen.

3 Herausforderungen bei klinischen Studien:
3 Herausforderungen bei klinischen Studien:
Kontinuierlich hochwertige Daten generieren
Die Abbruchquote reduzieren
Effizient kommunizieren
Wie SaniQ Sie dabei unterstützt:
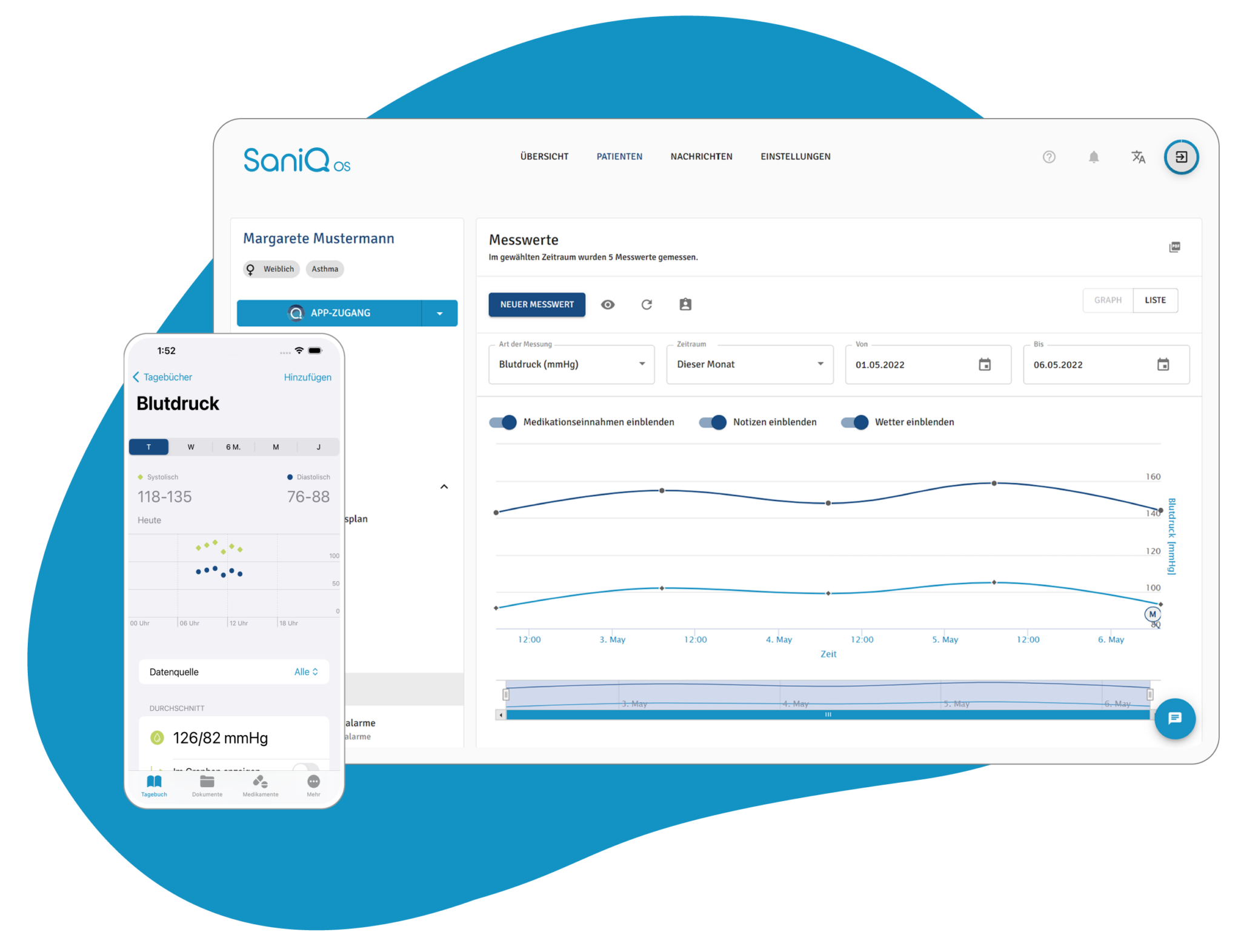
Was früher als isolierte Datenpunkte in sterilen Praxisräumen erfasst wurde, wird mit SaniQ zu einem lebendigen Bild des Symptomverlaufs im Alltag.
So begleitet SaniQ Ihre Proband:innen durch den Alltag:
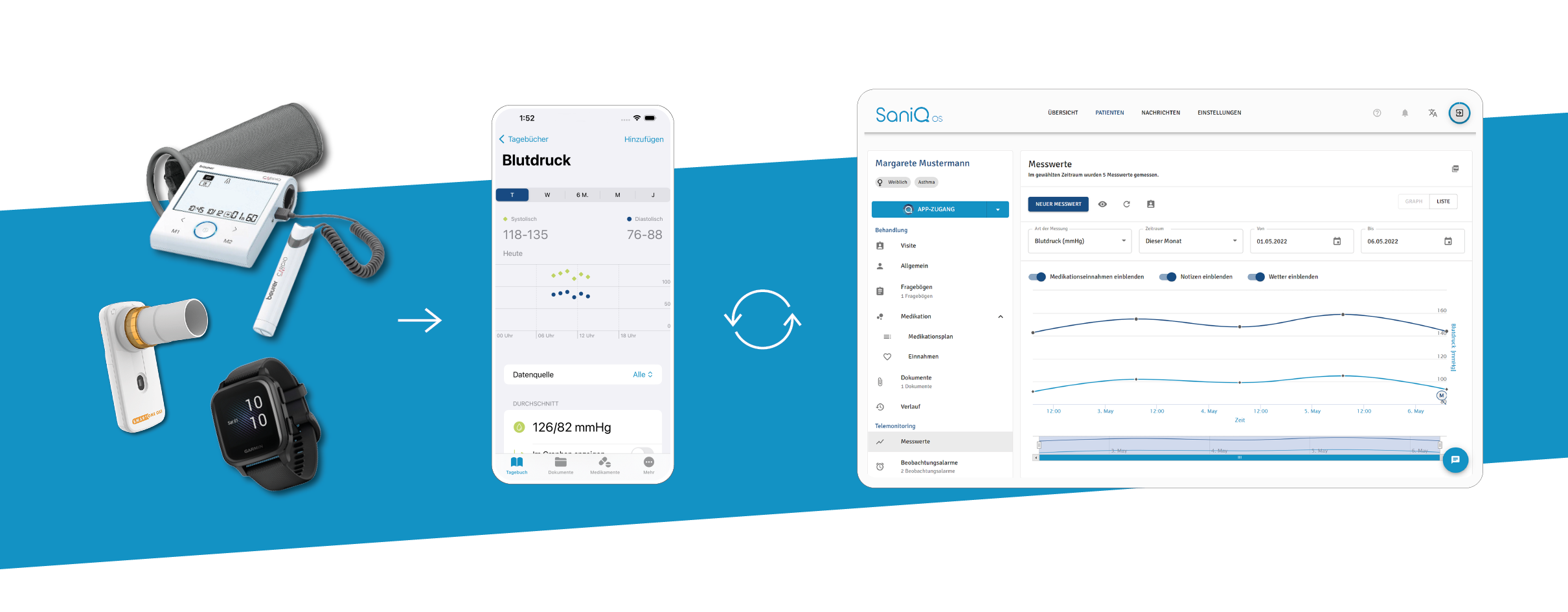
1. Morgens messen sie ihre Vitalwerte mithilfe an SaniQ angebundener Sensorik zu Hause.
2. Mittags beantworten sie digitale Fragebögen zu ihren Symptomen über die SaniQ Patientenapp.
3. Abends dokumentieren sie, wie sich die neue Therapie auf ihre Lebensqualität auswirkt.
SaniQ verwandelt so jeden Tag der Studienteilnahme in wertvolle Real World Daten, die Sie über das SaniQ Dashboard in Echtzeit verfolgen können.
Für Proband:innen wird die Studienteilnahme mit SaniQ wesentlich komfortabler:
Sie messen ihre Vitalwerte bequem von zu Hause, beantworten digitale Fragebögen (PREMs & PROMs), wann es ihnen passt, und erhalten sanfte Erinnerungen über die Patientenapp, wenn Sie mal etwas vergessen. Sollten doch einmal Fragen aufkommen, können sie sofort über die Plattform per Chat oder Videosprechstunde kommunizieren, statt auf den nächsten Termin zu warten.
Diese Flexibilität führt dazu, dass deutlich weniger Proband:innen die Studie vorzeitig beenden und die Datenqualität durch konsequentere Teilnahme spürbar steigt.

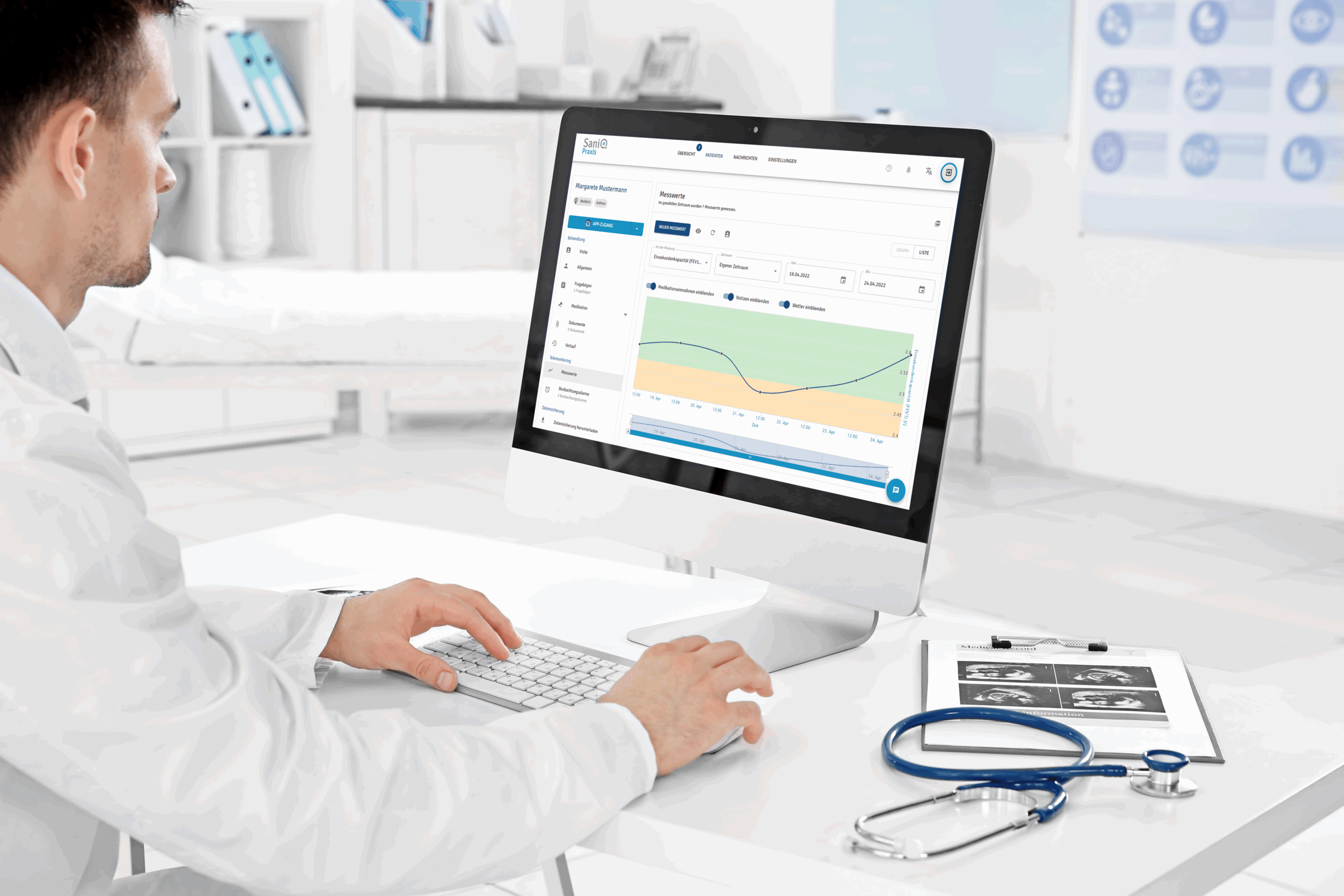
Treffen Sie Therapieentscheidungen, wenn sie gebraucht werden:
SaniQ benachrichtigt Sie direkt, wenn ein Proband auffällige Werte übermittelt. Mit wenigen Klicks können Sie daraufhin die aktuellen Vitalwerte einsehen, Fragebogendaten auswerten und per Chat oder Videosprechstunde das weitere Vorgehen besprechen, ohne dass der Patient erst einen Termin vereinbaren oder ins Zentrum fahren muss.
Diese schnelle Reaktionsfähigkeit ermöglicht es, die Therapie effizient und präzise an den Symptomverlauf anzupassen und gleichzeitig eine lückenlose Dokumentation für die Studiendaten zu gewährleisten.
1. Kontinuierliche Daten
So begleiten Sie Proband:innen mit SaniQ durch ihren Alltag:
- Morgens messen sie ihre Vitalwerte mithilfe an SaniQ angebundener Sensorik zu Hause.
- Mittags beantworten sie digitale Fragebögen zu ihren Symptomen über die SaniQ Patientenapp.
- Abends dokumentieren sie, wie sich die neue Therapie auf ihre Lebensqualität auswirkt.
SaniQ verwandelt dabei jeden Tag der Studienteilnahme in wertvolle Real World Daten, die Sie über das SaniQ Dashboard in Echtzeit verfolgen können.
Was früher als isolierte Datenpunkte in sterilen Praxisräumen erfasst wurde, wird so zu einem lebendigen Bild des Symptomverlaufs im Alltag.

2. Bessere Compliance
Für Proband:innen wird die Studienteilnahme mit SaniQ wesentlich komfortabler:
Sie messen ihre Vitalwerte bequem von zu Hause, beantworten digitale Fragebögen (PREMs & PROMs), wann es ihnen passt, und erhalten sanfte Erinnerungen über die Patientenapp, wenn Sie mal etwas vergessen. Sollten doch einmal Fragen aufkommen, können sie sofort über die Plattform per Chat oder Videosprechstunde kommunizieren, statt auf den nächsten Termin zu warten.
Diese Flexibilität führt dazu, dass deutlich weniger Proband:innen die Studie vorzeitig beenden und die Datenqualität durch konsequentere Teilnahme spürbar steigt.

3. Effizientere Therapiesteuerung
Treffen Sie Therapieentscheidungen, wenn sie gebraucht werden:
SaniQ benachrichtigt Sie direkt, wenn ein Proband auffällige Werte übermittelt. Mit wenigen Klicks können Sie daraufhin die aktuellen Vitalwerte einsehen, Fragebogendaten auswerten und per Chat oder Videosprechstunde das weitere Vorgehen besprechen, ohne dass der Patient erst einen Termin vereinbaren oder ins Zentrum fahren muss.
Diese schnelle Reaktionsfähigkeit ermöglicht es, die Therapie effizient und präzise an den Symptomverlauf anzupassen und eine gleichzeitig lückenlose Dokumentation für die Studiendaten zu gewährleisten.

- CE-approved Class I medical device
- GDPR-compliant data protection and ISO 27001-certified
- standardisierte Schnittstellen zu Ihrem KIS (HL7, FHIR)
-
CE-approved
Class I medical device -
GDPR-compliant data protection
and certified according to ISO 27001 -
standardisierte Schnittstellen
zu Ihrem KIS (HL7, FHIR)
Use Case: DIKAP-Studie
Die DIKAP-Studie „Digitale Kardiovaskuläre Prävention“ ist die aktuell größte Studie zur kardiovaskulären Primärprävention in Deutschland.
Period: 2025-2026
Partner: University Clinic for Cardiology and Angiology at Otto von Guericke University Magdeburg
Content: Mithilfe von SaniQ erforscht die Studie, wie digitale Lösungen zur Verhinderung von schwerwiegenden Herz-Kreislauf-Erkrankungen beitragen können.
Die Studie kombiniert dabei verschiedene SaniQ-Module:
- Digital training courses: Teilnehmende erhalten umfangreiche Informationen zu kardiovaskulären Risikofaktoren
- Telemonitoring: Überwachung von Blutdruck, Gewicht & körperlicher Aktivität
- Videosprechstunde: Regelmäßige telemedizinische Visiten
Heart attack & stroke: Can telemedicine better protect patients at risk?
Ein Forscherteam um Dr. med. Patrick Müller untersucht in einer neuen wegweisenden Studie am Universitätsklinikum Magdeburg, wie sich die Telemedizin nutzen lässt, um Risikopatienten besser zu schützen … weiterlesen
Studien mit SaniQ OS
SaniQ wurde bereits in zahlreichen klinischen Studien und Forschungsprojekten gewinnbringend eingesetzt:

Heart attack & stroke: Can telemedicine better protect patients at risk?
Ein Forscherteam um Dr. Patrick Müller untersucht, wie sich neue telemedizinische Technologien nutzen lassen, um die Versorgung von Risikopatienten zu verbessern.

Telemonitoring bei COPD in die Regelversorgung?
Telementor-COPD ist eine durch den G-BA geförderte, multizentrische, randomisierte und kontrollierte klinische Studie unter der Leitung der LungenClinic Großhansdorf. Sie untersucht mithilfe von SaniQ den Nutzen von Telemonitoring bei Patienten mit COPD.

Telemedizin schließt Versorgungslücke
Ein Ärztinnen-Team der Berliner Charité geht neue Wege, um den Betroffenen einer neurologischen Erkrankung die dringend benötigte Unterstützung zu bieten.
Das sagen Forscher:innen über SaniQ



Let's talk about your project idea!

Jonas Zimmer
-Project development-
Let's talk about your project idea!
- Personal counselling
- Individual topic focus
- Discover advantages